
While solid samples can be used for AAS this analysis is usually restricted to the more expensive graphite furnaces where the sample can be heated by controlled electrical heating as opposed to a direct flame. From here, the detector measures the intensity of the beam of light and converts it to absorption data. A monochromator is placed between the sample and the detector to reduce background interference. The light produced by the lamp is emitted from excited atoms of the same element that is to be determined, therefore the radiation energy corresponds directly to the wavelength absorbed by the atomized sample.

#Absorption spectra example free
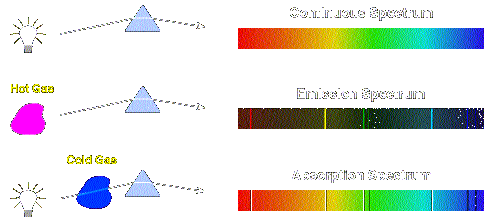
The free atoms are then exposed to light, typically produced by a hollow-cathode lamp, and undergo electronic transitions from the ground state to excited electronic states. In a typical experiment, the sample, either liquid or solid, is atomized in either a flame or a graphite furnace. A typical atomic absorption spectrometer consists of four main components: the light source, the atomization system, the monochromator and the detection system (Figure 1).įigure 1: Schematic diagram of a typical atomic absorption spectrometer. Furthermore, since the electronic structure of every element is unique, the radiation absorbed represents a unique property of each individual element and it can be measured.Īn atomic absorption spectrometer uses these basic principles and applies them in practical quantitative analysis. The radiant energy absorbed by the electrons is directly related to the transition that occurs during this process. When the atom is exposed to its own unique wavelength, it can absorb the energy (photons) and electrons move from a ground state to excited states. The electrons within an atom exist at various energy levels. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms.

When a sample containing copper (Cu) and nickel (Ni), for example, is exposed to light at the characteristic wavelength of Cu, then only the Cu atoms or ions will absorb this light. Firstly, all atoms or ions can absorb light at specific, unique wavelengths. The basic principles of AAS can be expressed as follows. Today, most analytical laboratories will boast at least one atomic absorption spectrophotometer. It took him several more years to convince manufacturers to use atomic absorption spectroscopy (AAS) for the detection of metals, but he eventually succeeded. By teatime on Monday morning, he showed that it could be done. Walsh decided to measure absorption, not emission. The normal procedure in spectroscopy was to vaporize an element and measure the emission spectra, but this technique was flawed and produced inaccurate results.
#Absorption spectra example how to
They were able to reproduce the black lines observed in the solar spectrum in the laboratory, thus allowing the identification of absorbing atoms in the corona through their emission spectra.Īlan Walsh, 2 a Lancashire-born physicist was working in his garden on a Sunday morning somewhere in the early 1950s when an idea that would solve a huge analytical chemistry puzzle popped up in his mind: how to accurately measure small concentrations of metallic elements by spectroscopy. Bunsen and Kirchoff demonstrated soon after that each chemical element had a characteristic color or spectrum when heated to incandescence (e.g., sodium (Na) yellow potassium (K) violet). In 1832, Brewster concluded that atomic vapors in the atmosphere absorbed some of the radiation from the Sun resulting in the detection of these lines. The English chemist, Wollaston, was the first to observe dark lines in the solar spectrum that became known as Fraunhofer lines. What are the applications of atomic absorption spectroscopy? Strengths and limitations of atomic absorption spectroscopy Interpreting an atomic absorption spectrometric output Radiation sources in AAS and signal detection Atomizing techniques - graphite furnace atomic absorption spectroscopy (GFAAS) - Atomizing techniques – specialized techniques Atomizing techniques - flame atomic absorption spectroscopy (FAAS) What is the principle of atomic absorption spectroscopy?


 0 kommentar(er)
0 kommentar(er)
